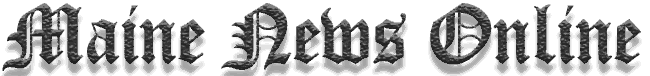![which is the noble gas notation for chlorine [ne] 4s2 4p5 [ne] 3s2 3p5 [ne] 3s2 3p3 [ne] 3p2 3p5 Decoding Chlorine's Electron Configuration](https://www.mainenewsonline.com/wp-content/uploads/2024/09/which-is-the-noble-gas-notation-for-chlorine-ne-4s2-4p5-ne-3s2-3p5-ne-3s2-3p3-ne-3p2-3p5-Decoding-Chlorines-Electron-Configuration.jpg)
The noble gas notation for chlorine is an important concept in chemistry, simplifying how we represent the electron configuration of atoms. Instead of writing out the full configuration, the noble gas notation uses a shorthand symbol representing the core electrons. For chlorine, which belongs to the halogen group on the periodic table, understanding this notation helps clarify its chemical behavior and how it bonds with other elements.
Chlorine has a total of 17 electrons, and to make its configuration easier, we reference the nearest noble gas (neon in this case), followed by its valence electrons. Throughout this article, we’ll answer the question: Which is the noble gas notation for chlorine? By examining options like [Ne] 4s2 4p5, [Ne] 3s2 3p5, [Ne] 3s2 3p3, and [Ne] 3p2 3p5, we will clarify the correct notation, explain the science behind it, and discuss how noble gas notation makes understanding chemical reactions more manageable.
In the following sections, we will break down each option and highlight why one notation is the most accurate representation for chlorine. Additionally, we’ll answer common questions and provide a detailed discussion of electron configurations in general.
Explaining the Correct Noble Gas Notation for Chlorine: [Ne] 3s² 3p⁵
To explain the correct noble gas notation for chlorine, we’ll break it down step by step to clarify the reasoning behind the electron configuration. Chlorine’s noble gas notation is [Ne] 3s² 3p⁵, and here’s how we arrive at this notation in a clear and numbered way:
1. Identify the Atomic Number of Chlorine
Chlorine has an atomic number of 17, which means it has 17 electrons. The electron configuration describes how these 17 electrons are arranged in different orbitals around the nucleus of the chlorine atom.
2. Find the Nearest Noble Gas
The noble gas notation is a simplified version of the electron configuration. To create the noble gas notation for chlorine, we use the closest noble gas that comes before chlorine in the periodic table. The nearest noble gas is neon (Ne), which has an atomic number of 10. This means neon has 10 electrons, and its electron configuration is 1s² 2s² 2p⁶.
3. Use Neon’s Electron Configuration as a Reference
Instead of writing out the full electron configuration for chlorine (1s² 2s² 2p⁶ 3s² 3p⁵), we can simplify the notation by using [Ne] to represent neon’s 10 electrons. Therefore, we don’t need to repeat the electron configuration for the first two energy levels, as this is already covered by neon’s configuration.
4. Add Chlorine’s Remaining Electrons
After accounting for the first 10 electrons from neon, chlorine still has 7 electrons left to place. These electrons will fill the 3rd energy level:
3s²: 2 electrons go into the 3s orbital.
3p⁵: 5 electrons go into the 3p orbital.
This completes chlorine’s electron configuration, resulting in [Ne] 3s² 3p⁵.
5. Understand the Importance of Valence Electrons
The 7 electrons in the 3rd energy level (2 in 3s and 5 in 3p) are the valence electrons. These electrons are responsible for chlorine’s chemical reactivity, as they determine how chlorine bonds with other elements. With 7 valence electrons, chlorine is highly reactive and tends to gain 1 electron to complete its octet, making it a common component in compounds like sodium chloride (NaCl).
By following this numbered breakdown, we see how [Ne] 3s² 3p⁵ is the correct and simplified noble gas notation for chlorine.
Key Noble Gas Notation Options for Chlorine
When determining the correct noble gas notation for chlorine, it’s essential to understand the structure of its electron configuration. Chlorine has 17 electrons, and the noble gas notation simplifies the full electron configuration by using the symbol of the nearest noble gas, neon (Ne). This notation helps to represent the inner, or core, electrons with the noble gas symbol, followed by the valence electrons of chlorine. Let’s explore the most common noble gas notation options and clarify which one is correct and why.
- [Ne] 4s2 4p5 – Incorrect Notation: This notation suggests that chlorine’s valence electrons occupy the 4th energy level. However, chlorine only has electrons in the 1st, 2nd, and 3rd energy levels. The 4th energy level is reserved for elements that appear later in the periodic table, such as potassium or calcium. Since chlorine’s electrons do not extend to the 4th energy level, this configuration is incorrect. Chlorine’s valence electrons are confined to the 3rd energy level, meaning the presence of “4s2 4p5” in this notation is misleading.
- [Ne] 3s2 3p5 – The Correct Notation: This is the correct noble gas notation for chlorine. It shows the electron configuration starting from the nearest noble gas, neon (Ne), which has 10 electrons. The next 7 electrons of chlorine are placed in the 3rd energy level, specifically in the 3s and 3p orbitals. This notation accurately represents chlorine’s electron configuration, with 2 electrons in the 3s orbital and 5 electrons in the 3p orbital, making a total of 17 electrons. This configuration is also important in explaining chlorine’s high reactivity, as it has 7 valence electrons and tends to gain 1 electron to achieve a stable octet.
- [Ne] 3s2 3p3 – Incorrect Notation: In this configuration, only 5 electrons are shown in the 3rd energy level, which means it inaccurately represents chlorine’s electron structure. Chlorine has 7 valence electrons in total, so this configuration falls short. The missing 2 electrons would make a significant difference in chlorine’s chemical properties, so this is not a valid representation.
- [Ne] 3p2 3p5 – Another Incorrect Option: This notation is incorrect because it suggests the duplication of orbitals. Electrons are distributed across the 3s and 3p orbitals, but this version inaccurately repeats the 3p orbital. As a result, this option does not accurately depict chlorine’s electron configuration.
The Wrapping Up
the correct noble gas notation for chlorine is [Ne] 3s2 3p5. This simplifies the full electron configuration of chlorine, making it easier to understand and apply, particularly in chemical reactions. Other options like [Ne] 4s2 4p5 and [Ne] 3s2 3p3 are incorrect because they either misrepresent the energy levels or the number of valence electrons. Noble gas notation is a valuable tool for chemists to quickly and accurately convey the electron structure of elements, making it crucial for predicting chemical behavior.
FAQ
What is the noble gas notation for chlorine?
The noble gas notation for chlorine is [Ne] 3s2 3p5.
Can chlorine have electrons in the 4th energy level?
No, chlorine only has electrons in the 1st, 2nd, and 3rd energy levels.
Why is [Ne] 3s2 3p3 incorrect for chlorine?
This notation is incorrect because it only shows 5 valence electrons, whereas chlorine has 7.